Intended Use
COVID-19 Ag Rapid Test Device is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human naso-pharynx. This test is for professional used only, as an aid to early diagnosis of SARS-CoV-2 infection in patient.
The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
* Specimen: Nasal Aspiration, Nasal Swabbing, Oropharyngeal Swabbing.
* Rapid test: 15 mins for results
*Simple operation
*Convenient storage: 2-30°C
*High sensitivity and specificity
*LOD: 400TCID 50/ml
Product Details
Principle The COVID-19 Ag Rapid Test Device uses double antibody sandwich immunoassay. The NC membrane pre-immobilized with monoclonal antibodies against SARS-CoV-2 antigen and anti-mouse polyclonal antibodies, and the colloidal-gold conjugated with monoclonal antibodies specific to SARS-CoV-2 antigen. If SARS-CoV-2 antigen present in the sample, a complex formed between the anti-SARS-CoV-2 conjugate and the antigen will be caught by the specific anti- SARS-CoV-2 monoclonal coated on the T region. Results appear in 10 to 20 minutes in the form of a red line that develops on the strip. Whether the sample contains the SARS-CoV-2 antigen or not, the solution continues to migrate to encounter another reagent (an anti-mouse IgG antibody) that binds the remaining conjugates, thereby producing a red line on the region C.
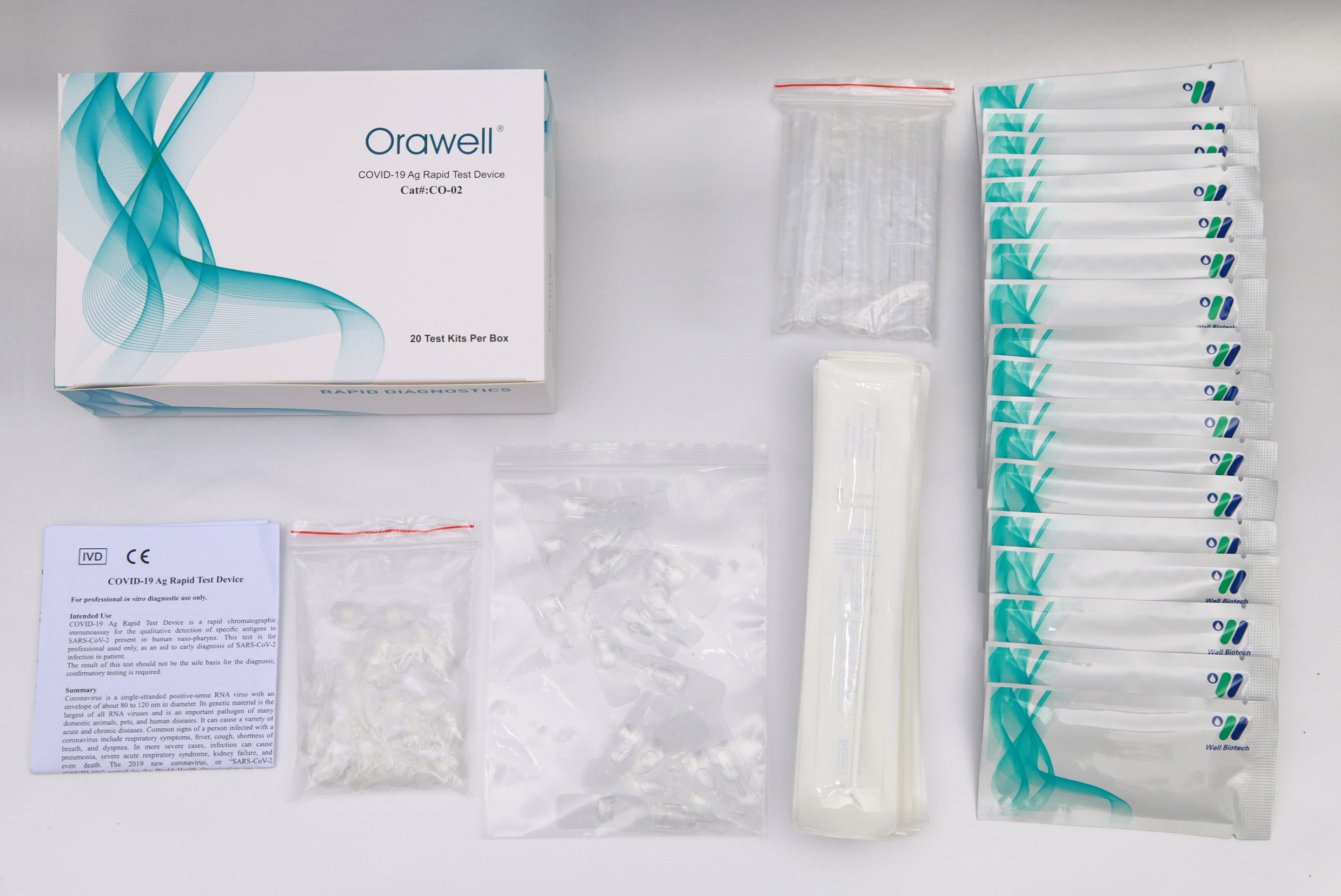
Materials Materials Provided
Test Device (individually packed in a foil pouch)
Sterilized Swab
Sample Collection Tube
Tube Stand
Sample Extraction Buffer
Package Insert
Materials Required but not Provided 1. Timer 2. Transfer pipette (for Nasal aspiration collection only)
Performance Characteristics
Clinical Evaluation
Relative sensitivity: 129/135= 95.6% (95%CI 93.79%~98.66%)
Relative specificity: 200/200 >99% (95%CI 97.12%~100%)
Overall agreement: (129+200)/(129+0+6+200)*100%=98.21% (95%CI 96.55%~99.36%)
CI: Confidence Interval
Copyright © Jiangsu Well Biotech Co., Ltd. All Rights Reserved. |
Sitemap
| Powered by
苏ICP备18032586号